DLP in NIRS: The Engine Powering Lab-Grade Precision Anywhere
Aug 06, 2025
Digital Light Processing (DLP) technology, pioneered by Texas Instruments, revolutionizes Near-Infrared Spectroscopy (NIRS) by replacing fragile mechanical gratings with 2 million micro-mirrors on a DMD (Digital Micromirror Device) chip. This innovation is the core of IAS analyzers, delivering unmatched reliability for industrial quality control.
DLP’s 3 Core Competitiveness
Extreme Stability:
Zero Moving Parts: Immune to vibration/humidity (vs. grating systems that drift in >80% RH).
Signal-to-Noise Ratio: >55,000:1 (industry avg. <30,000).
Impact: Stable operation on moving trucks or 45°C factory floors.
Lightning Speed & Precision:
Spectral Acquisition: 100 scans/sec (grating: 2-5 scans/sec).
Wavelength Accuracy: ±0.1 nm (critical for detecting trace adulterants).
Adaptive Intelligence:
Smart Pixel Control: Mirrors dynamically focus light on sample "hot zones".
Self-Calibration: Compensates for lens thermal drift (±0.001 nm/°C).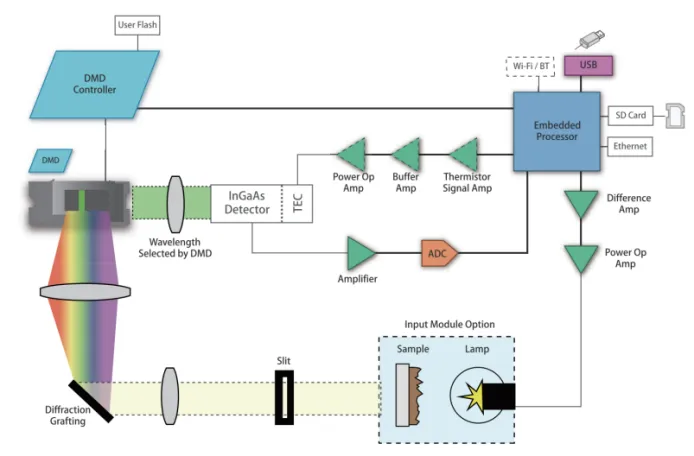
DLP vs. Grating: Key Performance Metrics
Parameter Traditional Grating DLP (IAS)
Scan Speed 5-10 sec/sample 0.3 sec
Humidity Tolerance Fails >80% RH 95% RH stable
Lifetime 2-3 years (mechanical wear) 10+ years (solid-state)
Resolution 8-10 nm <2 nm
Detection Limits Redefined
DLP enables IAS analyzers to achieve unprecedented accuracy:
For Oils (e.g., IAS-Olive™)
Free Fatty Acids (FFA): ±0.03% (EVOO threshold: 0.8%)
Polyphenols: Detect at 10 ppm (vs. HPLC’s 5 ppm)
Adulterants: Identify 3% lampante oil blends
For Solids (e.g., Grains)
Protein: ±0.15% at 30 sec/sample (ISO 20473 compliant)
Mycotoxins: Aflatoxin B1 LOD* <2 ppb
*Limit of Detection
Why Industry Leaders Choose DLP
Food & Agri: Unbreakable performance in dust/monsoon conditions (IP67).
Pharma: Meets FDA 21 CFR Part 11 for raw material ID.
Chemicals: Quantify trace impurities (e.g., sulfur in fuels <0.1%).
